Best Practices for Chemical Cleaning Hydroprocessing Units

Everyone knows a chemical cleaning vendor, and you may have met many different vendors who offer a variety of chemicals to help clean up your hydrocracker or hydrotreater prior to maintenance activities. In this article, we will discuss some of the available chemicals and how to improve the services obtained by chemical cleaning vendors. The intent is to provide a simple understanding of chemical cleaning for hydroprocessing units.
Keep it simple!
We have all had fun in the past with Rube Goldberg illustrations like the one above. He had a fantastic way of making simple things extraordinarily complex. Rube Goldberg devices do not belong in chemical cleaning, although I have certainly seen a few examples that imitate his machines!
The most important focus for chemical cleaning operations is to really keep it simple. All you are trying to do is clean out oil and pyrophoric materials (e.g., iron sulfide) to make the equipment safe to open and work on. Part of keeping it simple is to know exactly what work you need to complete prior to discuss it with a chemical vendor. Do you need to just blind off a portion of the unit or fully clean it to work on it? Is there hot work involved?
I have seen some chemical companies force refineries to construct elaborate, temporary blow-down headers that stretched the entire length of the unit and were connected to every low point. While blowing down critical low points can be an important part of chemical cleaning, some of these elaborate headers cost tens of thousands of dollars to build. A header like this may be necessary for a full unit turnaround, but not for a simple catalyst change.
Can you clean too much?
If you are not going to open it or work on it and it is not broken, you do not need to worry about cleaning it! Some chemical cleaning vendors are happy to take your money and clean up entire units when it may not be necessary. In some cases, such as furnace tubes, it is easy to cause damage by opening the tubes and exposing them to air and water, which can lead to polythionic stress corrosion cracking.
In one example, a unit decided to clean up the fuel gas piping. After not having plugged burners for years, they spent three months cleaning the liberated scale out of the burners before the header was fully cleaned up. The unit was limited to a low feed rate until the scale was gone.
An incident of overcleaning
In another unbelievably bad example, a refiner needed to just clean up a reactor, but the chemical vendor cleaned out and oxidized the entire high-pressure recycle loop. Cleaning of the piping and equipment in the recycle loop left piles of scale that washed into the reactor after the catalyst change causing a high pressure drop in the reactor (more on this example later in this article.
We should focus the cleaning on equipment that will be worked on and leave other equipment blinded under nitrogen to keep air and water out. Chemical cleaning vendors should be able to justify the cleaning they do rather than simply saying, “That’s what we normally do.” A good vendor should ask the question, “What do you need to clean?”
The basics: Why chemical clean equipment?
You know the reasons, but let us review the main goals. There are two primary problems that must be dealt with prior to opening and working on equipment: hydrocarbons and pyrophoric materials. Both hazards must be resolved to keep personnel safe and protect equipment while work is underway. One special case of chemical cleaning is removing LEL (Lower Explosive Limit) and other hydrocarbons from catalysts, which is discussed later in this article.
Hydrocarbon and hydrogen removal
It is necessary to remove hydrogen and hydrocarbons, particularly volatile hydrocarbons, to prevent explosions and fires while opening and working on equipment. Volatile oil or oil vapor is typically measured as a percentage of LEL. Hydrogen also shows up as LEL. At 100% or above LEL, the atmosphere inside the equipment may cause an explosion or fire when exposed to air. To be safe, most refiners set a maximum of no higher than 20% of LEL. Some refiners have lowered their LEL limit to 10%, but the ideal target LEL level is zero – meaning that all the volatile hydrocarbons were removed. Removing hydrocarbons often includes removing benzene to less than 1 ppm.
Often, LEL is measured in piping or a vessel that is under a nitrogen atmosphere. LEL measurements can be taken in nitrogen, but the instructions for the measurement device must be followed closely. Inert LEL measurements must be taken with a splitter device that draws a specific amount of oxygen in with the sample gas. Be sure to fully understand the instructions.
Fatal incident from failure to remove heavier hydrocarbons
It is important to remove volatile hydrocarbons to prevent detonation, but it is also important to remove all hydrocarbons to prevent fires. In one refinery, chemical cleaning was done to be able to pull bundles on a heat exchanger. After chemical cleaning, gas testing showed the LEL level in the heat exchanger to be extremely low and mechanics began to remove the flange bolts. The bolts were galled and difficult to remove, so they were cut with a torch since the LEL in the exchanger had been removed. During the bolt cutting, diesel leaked from the exchanger, resulting in a large fire and fatalities. The chemical cleaning did not remove the non-volatile oil. Remember that the lack of LEL is not a clear indicator of the lack of all hydrocarbons and most refiners will cold-cut bolts because of unknown environments that may be found within equipment. Heavier oils like diesel and gasoil may not contain volatile hydrocarbons that would be shown in a LEL test.
Pyrophoric removal
Pyrophoric or self-heating materials can build up in piping and equipment and may begin to smolder and then light on fire. Fire is a clear hazard to avoid, but smoldering and burning also remove oxygen while evolving carbon oxides (CO and CO2). These changes can turn an atmosphere that was assessed to be safe into a very hazardous environment for personnel.
In some cases, pyrophoric reactions can ignite other materials. There have been several incidents where iron sulfide in a distillation column reacted with air to start a fire. The fire caused the structured packing in the column to ignite, and the fire got hot enough to damage the column and cause part of it to tip over.

Iron sulfide has also been known to ignite catalyst (both fresh and spent). In some cases, the catalyst fire was so intense that the reactor had to be flooded with water to stop the fire. Catalysts must be kept in an inert environment or kept wet to avoid pyrophoric or self-heating reactions.
The bottom line is that pyrophoric and self-heating materials must be removed or neutralized before equipment can be safely entered and worked on, especially if hot work is to be done.
Why clean up equipment that you aren’t going to work on?
Remember that the piping and equipment that will not be worked on can be blinded and put under nitrogen to keep oxygen and water out. As an example, this is a good strategy for furnace tubes during a catalyst change. Unless the tubes need to be pigged for thickness measurements or for cleaning, keeping them in a nitrogen atmosphere will keep the inside of the tubes in good condition.
Another example of cleaning too much is neutralizing pyrophoric materials in the high-pressure recycle loop during a catalyst change. It is a necessary step to remove hydrocarbons by hot stripping the reactor loop, but the scale in the recycle loop piping does not need to be removed to safely blind the reactor and change catalysts. Unfortunately, I have seen chemical cleaning companies insist on cleaning everything in a high-pressure loop, even for a simple catalyst change.
What is the absolute best detergent for cleaning up oil?
Many years ago, an experienced chemist began to wonder about all the claims he had heard from chemical vendors. He listened to all the vendors state that their detergents cleaned up oil better than any of the chemicals their competitors offered. He saw that some chemical cleaning operations in the refinery worked very well and that some did not adequately clean refinery equipment.
He decided to compare the cleaning ability of all the detergents available from vendors in back-to-back, carefully controlled tests. He collected all the detergents, soaps, and similar chemicals from multiple chemical vendors. He then conducted several benchtop tests involving crude oil mixed with sand to assess the solvency of crude oil in a solution of each detergent and water. His test determined which detergent removed the most amount of crude oil from the crude/sand mixture when mixed well with each chemical and water.
After several rounds of careful testing, he was astonished at the result. Out of all the chemicals evaluated, the best soap was Dawn® Dishwashing Liquid!
When bench testing detergent chemicals, it may be wise to test the vendor chemical along with Dawn soap.

Chemicals need compatible procedures
It is an obvious statement, but even the best chemicals need proven procedures to be effective. No chemical will work well if used with the wrong procedure or if used outside the effective limits of the applied chemistry. Involvement of the vendor with proper experience is necessary for any chemical cleaning program to be effective. We encourage the involvement of vendors in all aspects of chemical cleaning with suitable skepticism about their claims. (Verify, then trust).
New chemicals and/or new procedures proposed by vendors MUST be vetted prior to use with proper Management of Change (MOC) procedures. Vetting should include bench testing, checking references with other refineries, and involvement of subject matter experts. We must be assured that any new chemical will solve more problems than it creates.
Common chemicals used in chemical cleaning
A simple chemical cleaning would use a detergent to remove hydrocarbons and an oxidizer to remove pyrophoric materials, if necessary. Let us look at some advantages and disadvantages of common chemicals used in chemical cleaning.
Detergents
These chemicals are used to remove hydrocarbons.
- Citrus-based detergents: Detergents containing terpene compounds such as limonene have been highly effective in cleaning up oil for decades. There are a variety of citrus-based detergents used by various vendors. The advantages of these types of detergents are:
- Can be used with both vapor-phase and liquid-phase cleaning procedures
- Inexpensive compared to other detergents; however, a few vendors have “proprietary” citrus detergents that can be exceptionally expensive
- Readily available
- Can be used along with other chemicals such as oxidizers without washing in between
- Performance is often enhanced by the addition of wetting agents, dispersants, and emulsifiers
- Enzyme-based detergents: Based on the combination of an enzyme and an amine oxide, these detergents have been shown to work well, but may not work as well as citrus-based detergents.

Oxidizer chemicals
These chemicals will be used to neutralize or remove or neutralize pyrophoric materials when necessary.
- Sodium carbonate peroxyhydrate: This is a common chemical that provides good pyrophoric removal. Its main advantage is that it does not leave a residue to clean up and it can be used at the same time as detergents. In my experience, this chemical is a particularly excellent choice.
- Potassium permanganate (KMnO4): Permanganates can be highly effective in pyrophoric treatment, but have a distinct disadvantage in that they leave purple residue behind that needs to be washed out. This chemical also cannot be used at the same time as a detergent.
- Sodium perborate: I do not have much experience with this chemical as there are only a few companies that use it. I am aware that it must be used in liquid-phase cleaning.
- Sodium nitrite: Sodium nitrite is a chemical that has only come into use in the refining industry over the past 20 years or so. This chemical cannot be used at the same time as a detergent.
- I have been involved in three different incident investigations where this chemical was used and the amount of scale generated during the pyrophoric neutralization was so great that the scale could not be adequately cleaned out. In all three incidents involving this chemical, scale that was not removed washed into the reactor upon unit startup, leading to a high pressure drop in the reactor. The most unfortunate part of these incidents is that the units were shut down for catalyst changes and a severe cleaning of the entire high-pressure recycle loop was simply not necessary. This is a classic case of cleaning “too much.”
- Unfortunately, I must recommend that you do not use sodium nitrite as it is simply too harsh. It cleans all the scale out of piping, which is not necessary. Cleaning all the scale requires that it be totally removed, which can be difficult.
Chemical cleaning to remove oil from catalyst
When changing catalysts, LEL, hydrogen, and total hydrocarbon should be removed from the catalyst and the reactor to safely dump catalyst, remove residual amounts of catalyst, and repair the reactor prior to loading fresh catalyst and putting the unit back in service. LEL and total hydrocarbon removal has historically been done with a “hot stripping” procedure.
Hot stripping removes LEL and hydrocarbon from the reactor through mass transfer. In other words, oil moves from an area of high concentration in the catalyst bed to an area of low concentration in the hydrogen recycle stream. Hot stripping is not a mechanical process where hydrogen pushes the oil off the catalyst.
To maximize the mass transfer rate of LEL and total hydrocarbon from the catalyst, the rate of hydrogen flow and the reactor temperature should also be maximized within reason. As I mentioned in a previous blog, the amount of time needed for hot stripping is cut in half for every 50⁰F increase in temperature.
In most cases, hot stripping is remarkably effective in removing LEL and total hydrocarbon from the catalyst. One situation where hot stripping can be less effective is when the reactor has substantial amounts of particulates or other materials that have caused a high pressure drop. In this situation, flow through the catalyst bed tends to channel, making the mass transfer operation more difficult. Difficult hot stripping can be overcome by increasing temperature and time for the hot strip.
The reason for discussing LEL and hydrocarbon removal is that chemical vendors are currently offering chemical treatment instead of hot stripping to remove LEL from the catalyst. The vendors still promote stripping, but at lower temperatures. They claim time savings over the typical hot strip procedure, but the chemical treatment can be excessively expensive.
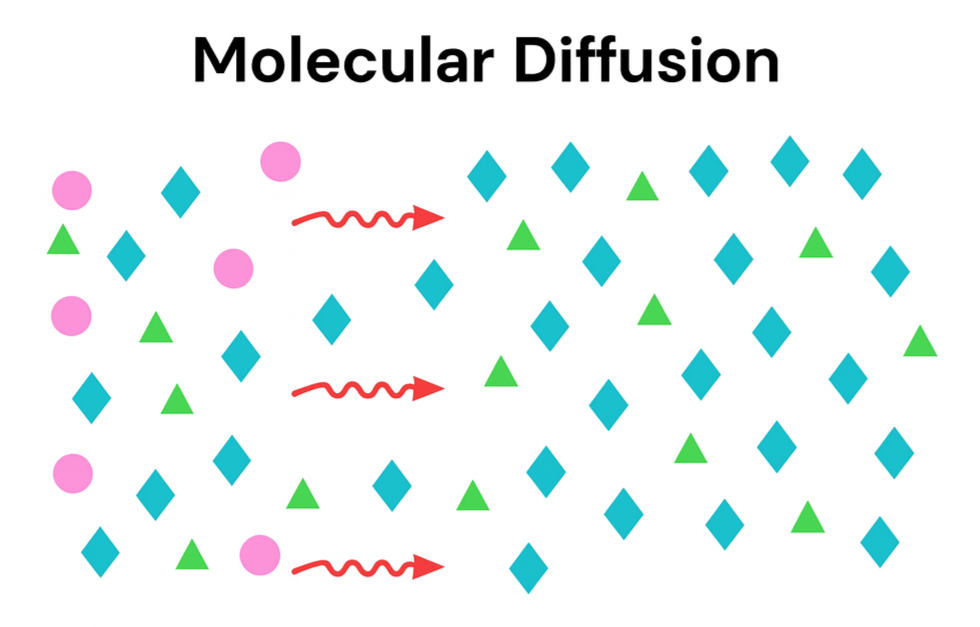
Mass transfer occurs solely because of the concentration difference between the two diffusing species at the two points.
Using chemical treatment instead of hot stripping
I took part in three different catalyst changes where the chemical treatment was used on a vacuum gasoil hydrotreater. Because the chemical was new to us, we still insisted that the catalysts be hot stripped both before and after the chemical treatment. This hydrotreater had a history of high pressure drop in the reactor, so we hoped that the chemical treatment would reduce the hot stripping time and the total time to shut the unit down.
In one of these cat changes, we watched the operation very closely to figure out the benefits of the chemical treatment. First, the catalyst was hot stripped until the bulk of the oil was removed. The reactor was then cooled down and the pressure lowered to be able to add the chemical in vapor phase to the reactor per the vendor’s procedures. Once the chemical treatment was completed, the reactor was heated up slightly for a final hot strip.
To our surprise, during the second hot strip, the separator on the unit began to fill with liquid. At first, the vendor claimed that the liquid was water, but a sample and analysis of the separator liquid confirmed that it was the chemical injected by the vendor. After hot stripping to remove the vendor’s chemical, we lowered the reactor temperature and were pleased to find that the LEL in the reactor was zero.
This experience taught us a few things. First, the chemical injected by the vendor appeared to help remove the LEL from the catalyst in a reactor that had been difficult to hot strip. Second, the chemical injected by the vendor stayed on the catalyst after the chemical injection process. Since the chemical was hydrocarbon, the residual amount remaining on the catalyst meant that some hydrocarbon had been left by the chemical injection process.
Therefore, if you use this chemical cleaning process instead of hot stripping, you must be aware that hydrocarbon will remain on the catalyst even if the LEL has been removed. As mentioned, catalysts are pyrophoric or self-heating. If the catalysts contain sulfides and begin to smolder, hydrocarbon left on the catalyst can create a huge fire very quickly, endangering workers that are removing catalysts and any other personnel who may handle catalysts before, during, or after shipping.
After this experience, we decided that hot stripping was much less expensive than the chemical injection process, even if the hot strip took more time. The only situation in which to consider a chemical aid might be to help a reactor with a high pressure drop. If a chemical is injected, it is wise to hot strip afterward to remove all hydrocarbon from the catalyst.
Vapor phase has many advantages
Vapor-phase cleaning has many advantages that should be considered and will save time. Vapor-phase cleaning uses fewer chemicals and therefore has less material to dispose of after the cleaning.
Once huge caution should be considered for hydroprocessing units: do not use steam in systems that are normally dry like the reactor loop! Introducing steam or water to a system that is normally dry will create substantial amounts of unnecessary scale. Using hydrogen or nitrogen is highly preferred as it keeps the systems clean and the gases can be disposed of to flare when done.
Circulation
Circulating the chemical cleaning in a loop or closed circuit rather than a once-through process can save a lot of chemicals and make the cleaning more efficient, especially with liquid-phase cleaning. Keeping circuits small can help cleaning efficiency. Handheld IR guns can help identify areas of piping or vessels that are at different temperatures from the rest of the system.
One value in a circulating system is the ability to reverse flow periodically to help dislodge solids and particulates.

Rumble!
One last word about an aid to chemical cleaning: adding turbulence to piping by “rumbling” nitrogen through it with the flow will help remove scale and other deposits. Rumbling nitrogen through a vessel will help to remove sludge in the bottom and loosen up deposits on internals.
Some vendors like to “rumble” the process with steam, but I have found that nitrogen is less likely to condense and creates more turbulence when rolling piping and vessels. Nitrogen also does not heat up the chemical cleaning solution. Having nitrogen available has an added benefit to preventing a system from pulling a vacuum when cooling down or emptying piping.
Application in your unit(s)
I hope that this article will give you more knowledge to properly deal with chemical cleaning vendors and to make good, economic decisions for your unit. A few takeaways:
- Choose a chemical vendor who will really add value to the process and is clear on the strengths and weaknesses of their chemicals and procedures.
- Collaborate with the chemical vendor to screen potential chemicals based on experience and references. Bench test the chemicals if necessary.
- Vendors and turnaround personnel should work together to create cleaning plans that effectively clean up the equipment and piping that will be worked on. Any equipment that will not be worked on can be blinded and blanketed with nitrogen to prevent air and water incursion. Do not clean more than is necessary.
- Create a procedure for hot stripping reactors to avoid unnecessary chemical cleaning expense for catalyst changes.
- Rumble your chemical cleaning circuits with nitrogen to increase effectiveness.
- Seek expert help to select contractors and chemicals that will be best for your unit.
- Last: keep it simple!
Until the next article – stay safe!
Like what you just read? Join our email list for more expert insights and industry updates.