Preventing Nickel Carbonyl Formation during Hydroprocessing Unit Shutdowns
The potential formation of nickel carbonyl is a hazard for all hydroprocessing units – both hydrocrackers and hydrotreaters. Fortunately, there have not been a lot of incidents in the refining industry related to nickel carbonyl, but there have been fatalities due to nickel carbonyl exposure. This blog will cover the risks of nickel carbonyl exposure, how it can be formed during a unit shutdown, and how to test to make sure it doesn’t form. I will also cover some myths that I have heard over the years about nickel carbonyl. Last, I will emphasize the importance of reducing CO concentrations before shutdown when processing renewable feedstocks.
Nickel Carbonyl Risks
100 workers at a Port Arthur, Texas petroleum refinery were exposed to nickel carbonyl many years ago. 31 experienced acute signs and symptoms of toxicity including headache, back and stomach pain, nausea, vomiting, chest constriction, shortness of breath, coughing, extreme weakness, and fatigue. Two workers subsequently died from this exposure. References for nickel carbonyl exposures are:
- Sunderman FW. A pilgrimage into the archives of nickel toxicology. Ann Clin Lab Sci. 1989 Jan-Feb;19(1):1-16. PMID: 2644888.
- Sunderman, F.W., Sr. 1992. Hazards from nickel exposure: A historical account. Pp. 1-20 in Nickel and Human Health: Current Perspectives, E. Nieboer, editor; , and J.O. Nriagu, editor. , eds. New York: Wiley.
I once met an operator who believed he was exposed to nickel carbonyl. His symptoms were consistent with exposure to nickel carbonyl and his doctors believed that exposure was the cause of his symptoms. He suffered long-term effects from his exposure that all of us would want to avoid.
Nickel carbonyl is an odorless (mostly), colorless liquid that is difficult to test for directly, which leads some people in the industry to downplay the possibility of formation and potential exposure. This article will show that it is relatively quick and easy to prevent nickel carbonyl formation in hydroprocessing units.
Nickel carbonyl is one of the most toxic compounds used in industrial applications. The toxicity and morbidity are comparable to hydrogen cyanide. Acute nickel carbonyl poisoning – ScienceDirect
| US Health Exposure Limits (NIOSH) | |
| Permissible Exposure Limit | 1 ppb |
| Recommended Exposure Limit | 1 ppb |
| IDLH (Immediate danger) | 2 ppm |
Nickel carbonyl forms from a reaction with finely divided or powdered nickel metal and carbon monoxide:
Ni(s) + 4 CO(g) → Ni(CO)4(g) Nickel Carbonyl data sheet
Nickel tied up in metal such as stainless steels and chrome moly steels does not react to form nickel carbonyl. Nickel oxides and sulfides on catalyst are finely divided enough to reactor with carbon monoxide to form nickel carbonyl.
Nickel Carbonyl Formation during Hydroprocessing Unit Shutdowns
Nickel carbonyl decomposes at temperatures around 300°F (150°C), so we can avoid creating more than 1 ppb of nickel carbonyl if reactor temperatures are maintained above 400°F (200°C) until the concentration of carbon monoxide in the gas flowing through the reactor is below the concentration threshold that will form <1 ppb (0.001 ppm) in the reactor.
In most reactors, a target CO concentration of less than 10 ppm will ensure that no more than 1 ppb nickel carbonyl can be formed. In some reactors which operate at high pressures and low temperatures (shown above the PEL line in the chart below), it may be necessary to require a CO concentration limit that is less than 10 ppm to prevent nickel carbonyl formation
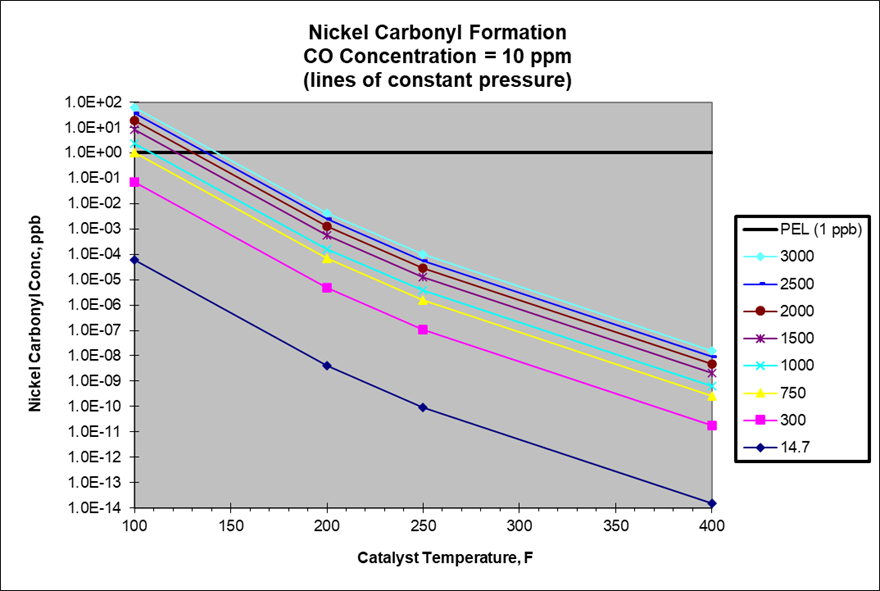
Correlation for Predicting Nickel Carbonyl Formation based on CO Concentration
Substantial kinetic data has been produced on the kinetics of nickel carbonyl formation. One source of kinetic data is found at this link: kinetic data on nickel carbonyl formation.
The following equation has been used for many years to predict the concentration of nickel carbonyl formed at various pressures and temperatures. This equation was used to produce the chart listed above.
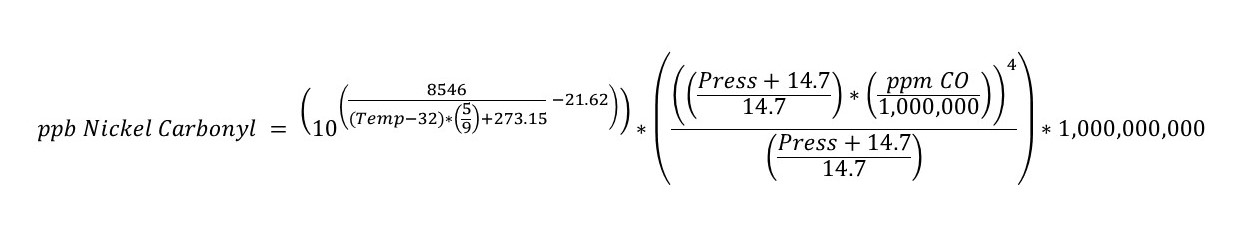 Where: Temp = temperature in degrees F
Where: Temp = temperature in degrees F
Press = pressure in psig
ppm CO = parts per million CO measured in the gas flowing through reactor
Testing for Carbon Monoxide during a Hydroprocessing Unit Shutdown
- DO NOT use an electronic testing device to measure low levels of CO in the gas streams. While these instruments are useful for higher levels of CO, they are not accurate near 10 ppm. Many units have delayed their shutdowns by mistakenly using electronic measurement units to try and measure CO in the 0-10 ppm range.
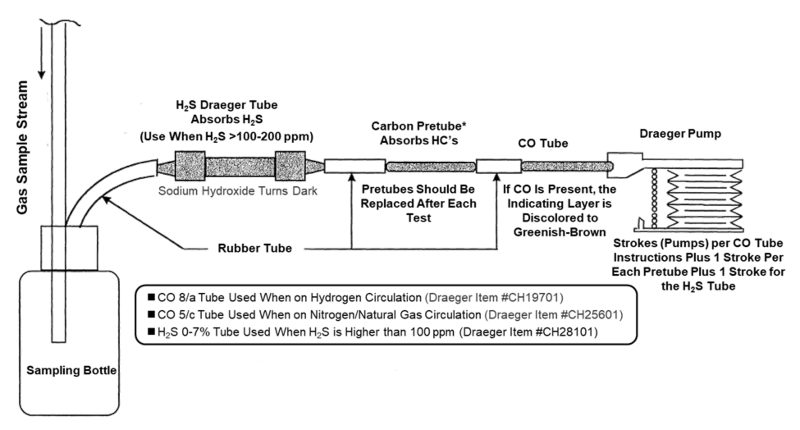
- USE a Draeger (or equivalent) testing tube with the proper procedure to test for CO. The diagram to use a Draeger testing tube is shown below. Note that different Draeger tubes are necessary for >50% hydrogen gas and >50% nitrogen gas.
- The H2S and carbon pre-tubes are necessary to avoid interference readings
- The bottle is necessary to prevent liquid from contaminating the tubes
- The tubes should be stored at room temperature to make sure the reaction that takes place in the tubes will occur normally
- The tubes have an expiration date – do not use old tubes.
- Test both the makeup H2 and the recycle H2 for CO and do repeats of each test
- If you have the tubes and testing apparatus, this test takes less than 5 minutes. It’s certainly worth 5 minutes to prevent fatal exposure to nickel carbonyl!
- If the CO concentration is greater than 10 ppm (or the limit predicted by correlation above), then high CO gas should be purged out of the system before cooling down below 400°F (200°C).
- Getting rid of high CO gas may be done by switching hydrogen sources or changing the operation on the hydrogen unit to reduce CO content.
- An alternate solution to purging high CO concentration in the hydrogen is to switch to nitrogen gas before cooling down.
- The nitrogen gas used to displace hydrogen should be tested to make sure it is less than 10 ppm CO. Some nitrogen supplies can exceed 10 ppm!
- See the diagram below for further instructions on testing.
Myths about Nickel Carbonyl Formation
- I don’t need to test because I don’t have nickel catalyst in my reactor – False!
- This myth is false!
- Almost all reactors have nickel catalyst present – many types of grading catalyst and support catalyst contain nickel
- Catalyst bags can be mislabeled, allowing nickel catalyst to be loaded when it was not suspected
- Catalysts that are not supposed to contain nickel such as cobalt moly catalysts are produced in the same facilities as nickel-containing catalysts. Have you ever seen the following label on food?

- This label is particularly important for those who have nut allergies. There should be a similar warning on all catalysts, “Manufactured on the same equipment that processes nickel catalyst!”
- Nickel oxide catalyst will not form nickel carbonyl in the presence of CO.
- This myth is false!
- Any finely divided nickel surface will produce nickel carbonyl in the presence of CO
- Nickel catalyst will not form CO in our reactor due to the presence of “X.” I have heard many claims that nickel carbonyl formation is prevented by the presence of some gas like oxygen, H2S, etc.
- The myth is false!
- Other species present will not prevent the formation of nickel carbonyl – only an extremely low concentration of CO will prevent forming more than 1 ppb.
- We have never seen nickel carbonyl in any of our reactor shutdowns and catalyst dumps, so we should not expect it in our unit
- This myth is false!
- How would you know that you have never seen a colorless and mostly odorless liquid until someone develops symptoms of exposure
- Testing for nickel carbonyl is exceedingly difficult
- This myth is true due to both sample collection and the required analytical procedure
- BUT – testing for CO is quite easy and simple
- Isn’t it worth 5 minutes to test for CO and prevent a potential fatality?
- Side note: There is a multi-step wet chemistry method for testing for nickel carbonyl directly, but it is time consuming and difficult. Why make it hard when testing for CO is extremely easy?
- We only need to test for CO during planned shutdowns – never for emergency shutdowns
- This myth is false!
- I can’t count the number of times that a unit has had an emergency shutdown and then had to go into the reactor. Emergency shutdowns can cause increased pressure drop and other difficulties that may require reactor entry.
- If you keep the Draeger tubes (or equivalent) on hand with the test equipment, it only takes 5 minutes to conduct the test – just in case something happens that you need to enter the reactor.
Special concerns when processing renewable feedstocks
High levels of oxygen and other contaminants in renewable feeds form CO as a byproduct when processed in a hydroprocessing unit. Some processing of renewable feedstocks can lead to a percent-level concentration of CO in the recycle gas loop.
Therefore, it is necessary to stop feeding renewable feedstocks at least a few days before a shutdown to stop forming CO. The CO present in the recycle gas should be fully purged out of the reactor system prior to cooling down below 400°F. Your catalyst vendor/licensor may have additional recommendations on how the shutdown procedure should be performed to prevent nickel carbonyl formation.
Application in your unit(s)
- Does your unit have clear procedures to prevent nickel carbonyl formation during shutdown?
- Does your refinery and unit stock the test tubes and equipment to test for CO to prevent nickel carbonyl formation?
- Are your personnel trained on the hazards of nickel carbonyl and the procedures to use to prevent its formation?
As always, we welcome and appreciate feedback, questions, comments, and suggestions.



